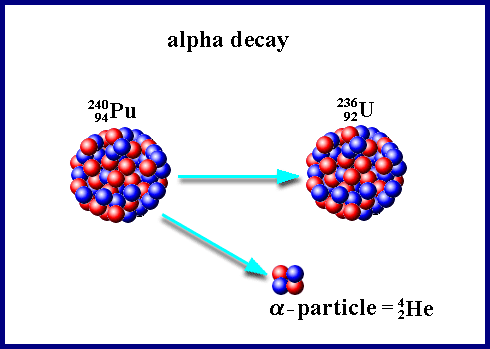
6 Links that helped me:
Stellar-Database- This link basically gave me my whole Elements In The Stars project and tied up anything I couldn't find on a star.
BBC- Although this website is mainly to explain how Nuclear Bombs work, it does highlight and explain some points we are working on in our unit.
Introduction to Atomic Physics- Yes, this link will take you to a physics website, but it covers our entire unit in detail. In my opinion, one of my favorite websites for this topic.
Atomic Structure and Radioactivity- It's like the title says. This website is best for when your low on time and need a site that divides the information into a table of contents to conserve time.
BBC #2- This site does a complete run-through of the subject and offers links that you can click on if you need more information.
A Simple View of Atomic Structure- This site offers what the title says, "A Simple View of Atomic Structure". It runs through the sections of the modern atomic theory and hits on the main points.