Links:
Monday, May 9, 2016
Air Bag Lab
Gas Laws Lesson
 |
| Equation |
Gas Laws lesson 1
 |
| Equation |
Sunday, April 24, 2016
Energy Unit Test
 |
| We all did good! |
 |
| How I feel I did |
Specific Heat of a Metal Lab
We did a lab to measure the specific heats of copper and lead by heating the metals in a hot water bath and figuring out the variables for MCAT. Overall Liesel and I were sastified because we yielded only a 4% error. Now for Dylan Kossl I can't say he yielded a good percent error *cough cough* like 120% error *cough*. Here is a video of our test tube making a popping sound.
Here is some pictures of the copper in the test tube.
 |
| Side view |
 |
| Top view |
First Lecture On Energy
 |
| Energy equation |
 |
| Endothermic Reaction |
 |
| Exothermic Reaction |
Mrs. Frank also specified that we can't just stick a thermometer into a system, so whenever we read the temperature we are reading the temperature just outside the system. She introduced to us the equation of Q = mcΔT which we then put into practical use and calculated energy resulting after bonds have reformed. Overall I thought it was very easy. I am going to practice a few problems tonight just to make sure I have it down.
Friday, April 22, 2016
Race Day!
Today we raced our boat. Last night I was in a rush and didn't have time to glue my first boat together. So this morning I grabbed the simplest and lightest boat I made. Shown below
I thought our boat was going to be the slowest and was relieved to find out that was not true. That would be Dylan Kossl's boat. Shown below
After my first attempt I realized that my belief was drastically inaccurate, our boat was the fastest. My first run timed in at 12.1 seconds. I went again and timed in at 10.39 seconds.
I thought I didn't set my motor up right so I re raced unofficially with a new method. Mrs. Frank timed it at 8.95 seconds! She told us that was the fastest all day! The video is below.
 |
| didn't have time to finish gluing |
I thought I didn't set my motor up right so I re raced unofficially with a new method. Mrs. Frank timed it at 8.95 seconds! She told us that was the fastest all day! The video is below.
Tuesday, April 12, 2016
Building of the Biodiesel Putt Putt Boat
 |
| A design I am considering |
| Another design I considered |
Thursday, April 7, 2016
Biodiesel Video
 |
| Biodiesel Bruh |
 |
| Green stuff going on |
Wednesday, April 6, 2016
Unit Test for Chemical Bonding
 |
| Meh |
 |
| MEH |
Lesson About Chemical Bonding
 |
| Lewis dot stuff |
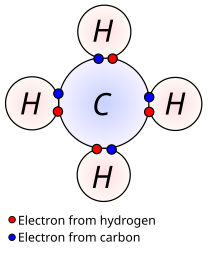 |
| Bonding bruh |
Monday, March 7, 2016
Electronic Structure and Periodic Trends Unit Test
| Meh |
Okay I'm back and I have taken it. It was harder than I thought, but it wasn't too terribly hard. I was able to finish in the time given which is always a plus. I ended up getting an 88% which I think isn't bad, but not satisfactory. I am happy about how I didn't fail though..... Onto the next test.
Periodic Trends Lesson
 |
| Periodic Trends |
Thursday, March 3, 2016
Wednesday, March 2, 2016
Weekly Quiz 1
 |
| : ( |
Tuesday, March 1, 2016
Electronic Structure and Periodic Trends Lab
I don't exactly see the point of the lab to be honest. I spent the entire hour adjusting a little knob that with the slightest twitch will jump up past what I need it at. Me and Matt shared our machine with another group and so I really should be trying to understand what the meaning behind the lab is, but I really don't feel like it. This entire paragraph is just afiller and Ihave no idea what to say about this lab except I should be trying to understand it. It was just testing different substances on how they respond to light. Anyway here is a picture:
 |
| Stuff is happening |
First Lesson On Electronic Structure
 |
| Periodic Table |
Thursday, February 18, 2016
UNIT TEST
 |
| Ok? |
Tuesday, February 9, 2016
Titration Lab 2
 |
| Unknown Acid done mixing |
 |
| Unknown Acid Being Dissloved |
This lab was basically the same thing of the last lab. The only thing we did different was use an unknown acid. We finished the lab with a very small percent error and ended up getting a 29.5 out of 30. We were lucky because we got the solution that allowed us to do minimal work. We cut our work in half and finished early and had time to work on our note card. We did struggle with the note card because I did have the best knowledge of what exactly we were doing, but we eventually figured it out. We started by writing out the chemical formula and balancing it. Then we plugged and chugged and ended up with the mols of HA and then divided it by the grams of the unknown substance.
 |
| Titrated KHP |
Sunday, February 7, 2016
Percent Acid in Vinegar Lab
The Lab was fun actually, but very tideous. The problem was that 1 drop difference, literally a 1 drop difference. If you add just one extra drop it will turn a bright pink. It was a great example of the equivalence point. The point where the solution goes from an acidic solution to a basic solution. We did have to make several runs at it though because of that 1 drop difference. If that doesn't tell you, we failed several times and made a very bright pink solution.
 |
| Our Setup |
 |
| Finally got it |
Friday, February 5, 2016
Lesson on Acid-Base
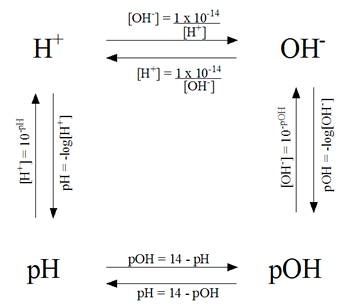 |
| Conversion Chart |
Acid-Base Reactions Quiz
 |
| Thumbs up |
Monday, January 25, 2016
Aqueous Solutions Unit Test
Took the unit test today. I think I did really good. I studied for a very long time and did as many practice problems as possible and I think it all paid off. I read other people's blogs and I don't know how I feel about the grade I achieved but I know I didn't fail so that's always good.
The test took all hour and each question was lengthy. It was a mix of stoichiometry, m1v1=m2v2 problems and a lot of morality problems.
The test took all hour and each question was lengthy. It was a mix of stoichiometry, m1v1=m2v2 problems and a lot of morality problems.
 |
| Test |
Thursday, January 21, 2016
KMnO4 Lab
 |
| *nudge* |
Friday, January 8, 2016
Lesson About Aqueous Solutions
 |
| Not Bad |
Subscribe to:
Comments (Atom)